Best Solvent for Benzoic Acid Recrystallization
The purity of benzoic acid depends on how large the range of the melting point is. Dingxin Liu Crystal Growth Design Articles ASAP Article Publication Date Web.
Solved Based On The Structure Of Benzoic Acid List Three Chegg Com
MIL-101Fe-Attached Graphene Oxide for High-Performance Supercapacitors with Sound Stability in Acid Electrolyte.
. Compositional Control and Optimization of Molecular Beam Epitaxial Growth of. 0 C 35 40 111 35-60 The boiling point of the recrystallization solvent should be lower than the melting point of the compound to be recrystallized. An isolated yield of 77 was achieved with acetic acid as the solvent.
Of benzoylformic acid was used a. A To purify samples of organic compounds that are solids at room temperature b To dissociate the impure sample in the minimum amount of an appropriate hot solvent Equipment Materials. Pahlavan 3 Example 1- The solubility of solid X in hot water 550 g100 ml at 100 oC is not very great and its solubility in cold water 053 g100ml at 0 oC is significantWhat would be the maximum theoretical percent.
NOMENCLATURE IN ORGANIC CHEMISTRY Contents 1. Recrystallization and Melting Point EXPERIMENTAL PROCEDURE. By choosing a suitable recrystallization solvent and following the steps above separation of a substance and any impurities can be achieved.
Predict the organic product of the following reaction h2so4. The ROP was quenched with benzoic acid and the solution was precipitated in cold MeOH 0 C. Using a hot plate dissolve approximately 10 g of impure benzoic acid in 30 35 mL of hot water water at or near its bp in a 125 mL Erlenmeyer flask.
Refer to Zubrick text for additional details Recrystallization. Besides reading the lab manual you will need to do a little bit more. Benzoic acids melting point range from 121 C- 125 C meaning it have 4 potential melting ranges that could be identify as benzoic acid.
Ketoxime ether 1 010 mmol aroylformic acid 2 030 mmol. Solvents Used Mass of Crude g Mass of Recovered g Amount of Solvent Used mL Percent Recovered Experiment One Water H2O 051 048 10 941 Experiment Two Methanol MeOH And Water H2O 049 045 Methanol 15 Water 05 92 Conclusion Both experiments were of fair solubility but in the case of recrystallization of Benzoic Acid Water was the best solvent. Further recrystallization was conducted from ethyl acetate-hexane to provide O-methyl ketoximes.
If there is a residual amount of material. 1 H NMR PS-b-PDLA 400 MHz CDCl 3 25 C. CHCH 3COO 156215 1H.
CH 2 CHPh 152166 d 3H. An ideal recrystallization solvent can fully dissolve a substance and impurities at high temperature but cannot fully dissolve the substance at cooler temperatures. General Procedures for Ortho-Benzoylation of Acetophenone Oxime Ether.
Crystallization or recrystallization is the most important method for purification of organic compoundsThe process of removing impurities by crystallization involves dissolving a compound in an appropriate hot solvent allowing the solution to cool and become saturated with the compound being purified allowing it to crystallize out of the solution isolating it by. CHEM 2423 Recrystallization of Benzoic Acid Dr. To help you understand the chemical basis of this exercise you should review Sections 35 - 37 in Solomons Fryhl which concerns the.
The narrower the range the more pure the compound is and the wider the range the less the purity of a compound. Because of this we do not allow traffic to our website from outside the UK so unfortunately you will. Of the length of the inner pipe.
CHEM 2423 Recrystallization of Benzoic Acid Dr. PS- b-PDLA was isolated by centrifugation and dried in vacuo for 24 h at 40 C. Explain why the Grignard reagent is more reactive.
The other reaction products including the magnesium bromide will remain in the aqueous layer clearly showing that separation based on solubility is achieved. Benzoic acid is more soluble in an organic solvent such as dichloromethane or diethyl ether and when shaken with this organic solvent in a separatory funnel will preferentially dissolve in the organic layer. Chem-2423-recrystallization-of-benzoic-acid-dr-pahlavan 22 Downloaded from aghsandbox.
Liquidliquid extraction LLE also known as solvent extraction and partitioning is a method to separate compounds or metal complexes based on their relative solubilities in two different immiscible liquids usually water polar and an organic solvent non-polar. Junjie Xie Rui Ma Haobin Fang Haoran Shi and. There is a net transfer of one or more species from one liquid into another liquid phase generally from aqueous to.
It can take quite a while to be completely dry and trying to remove crystals when there are still traces of solvent will kinda. CH 2 CHPhCH 2 366389. The magic behind the app is a set of Language Models.
Pahlavan 1 EXPERIMENT 4 - Purification - Recrystallization of Benzoic acid Purpose. We notice you are outside the United Kingdom. Of course its still likely that an examiner will give you an amino acid with more than one acid group and ask you to Aug 04 2021 Organic chemistry is the study of the structure property and reactions of organic compoundscompounds that contain carbon.
After the metal will be cooled through different mediums air oil water or other substances. Rhombic calcites grow in low. δ ppm 041067 8H.
At the moment we only ship our products to addresses in the UK.

Recrystallization Choose The Most Appropriate Solvent To Obtain A Successful Recrystallization Of Benzoic Acid Gcse Science Marked By Teachers Com
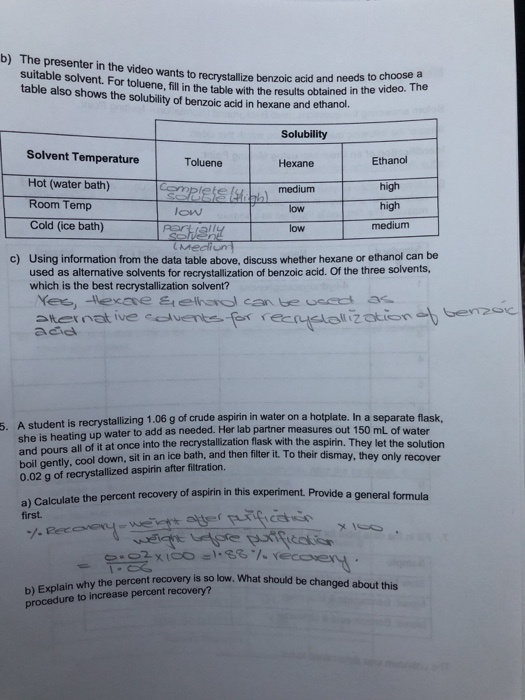
Solved B The Presenter In The Video Wants To Recrystallize Chegg Com

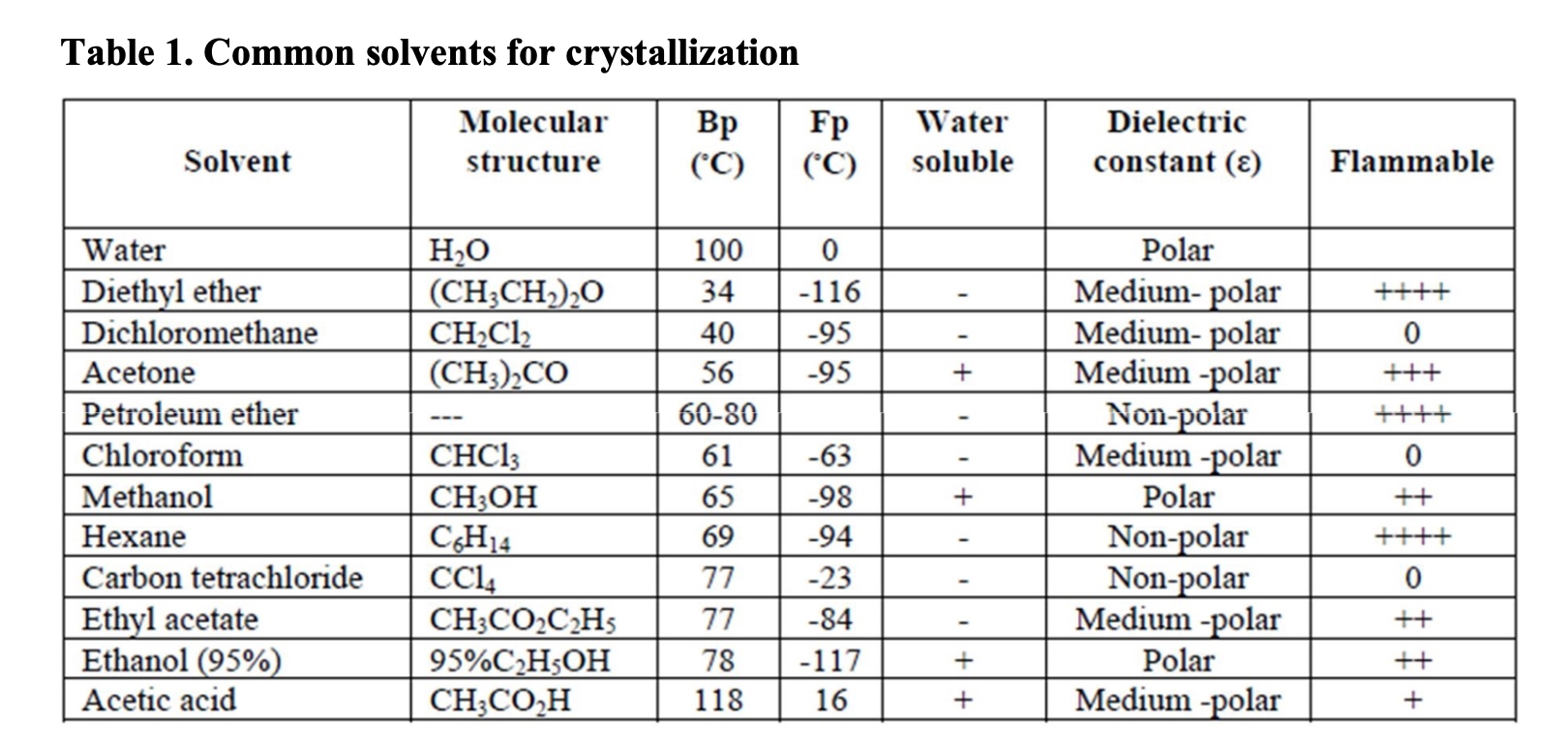
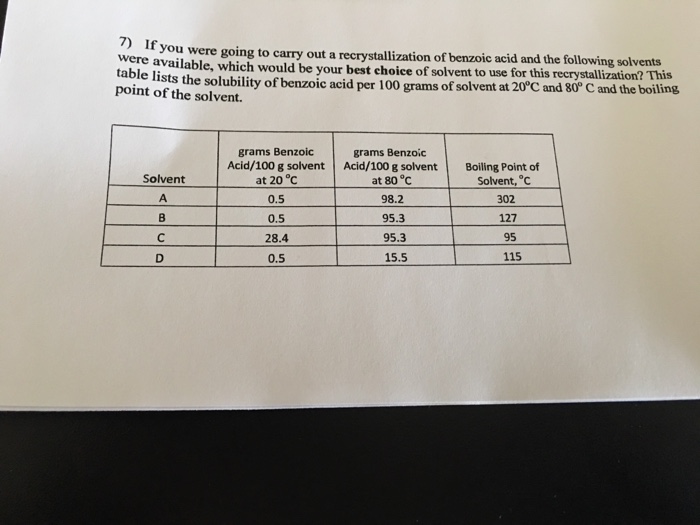
Solved 7 If Wee Alable Which Would Be Your Best Choice Of Chegg Com
0 Response to "Best Solvent for Benzoic Acid Recrystallization"
Post a Comment